
Cephalopods were mollusks, like gastropods, scaphopods, chitons, and bivalves. Like many mollusks they had shells made of calcium carbonate and processed food with a toothed, tongue-like radula. Cephalopods are still alive today, and include the nautilus, squid, cuttlefish, octopuses, and others.
Cephalopods found at Seven Stars
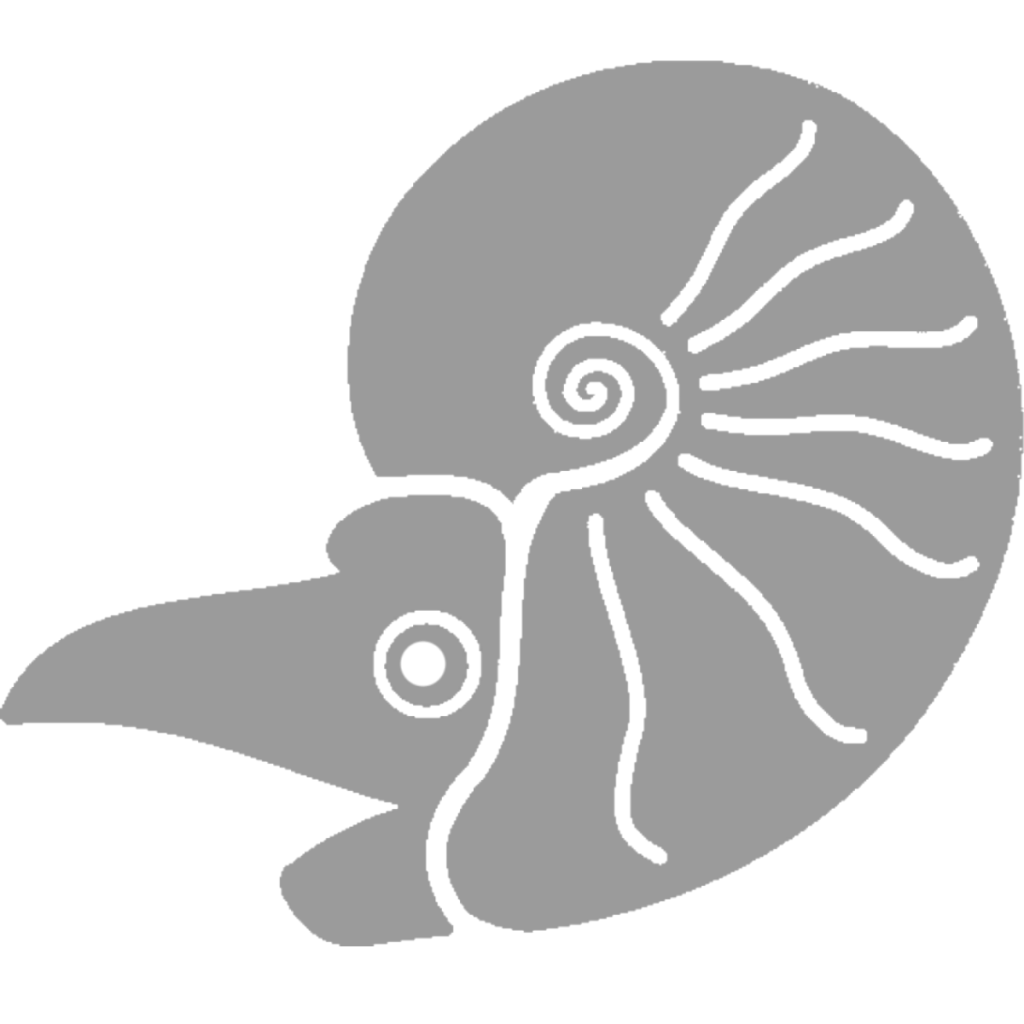
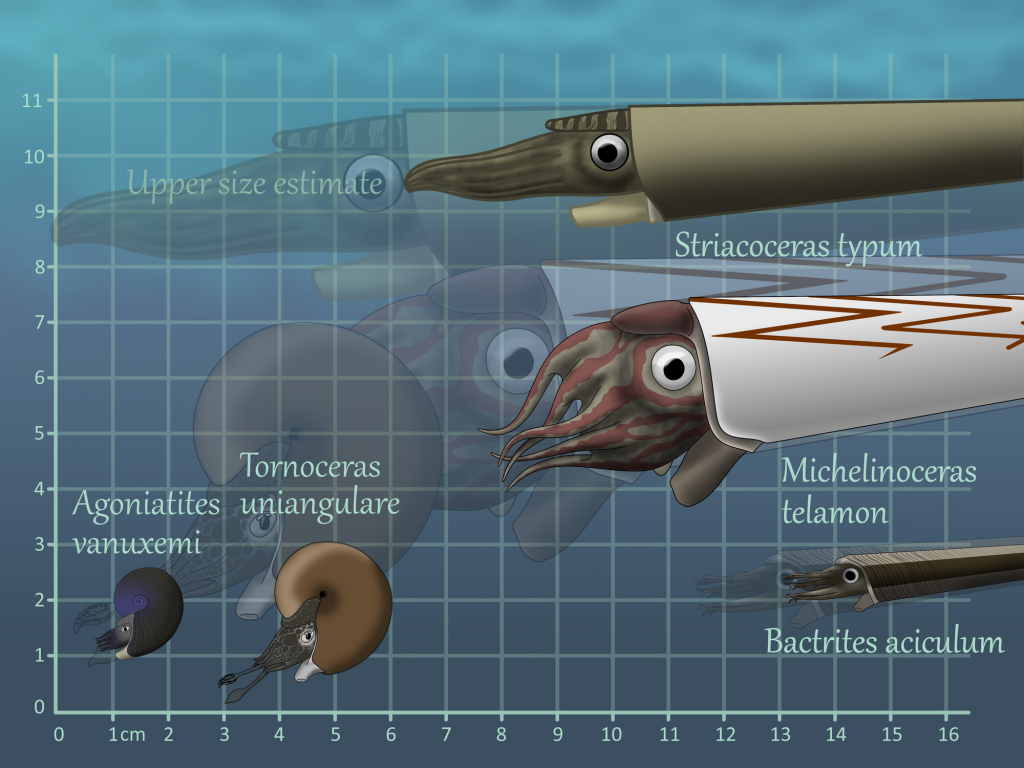
Agoniatites vanuxemi
VERY RARE
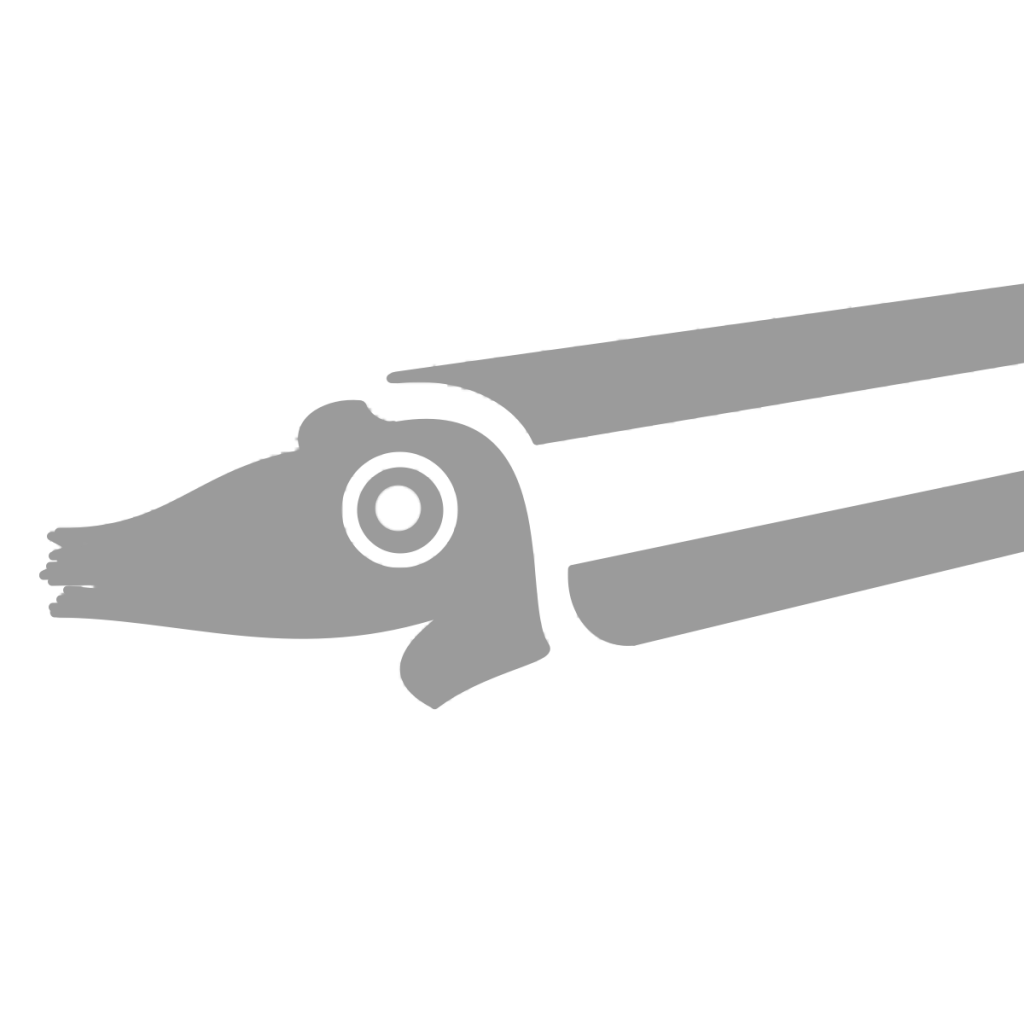
Bactrites aciculum
UNCOMMON
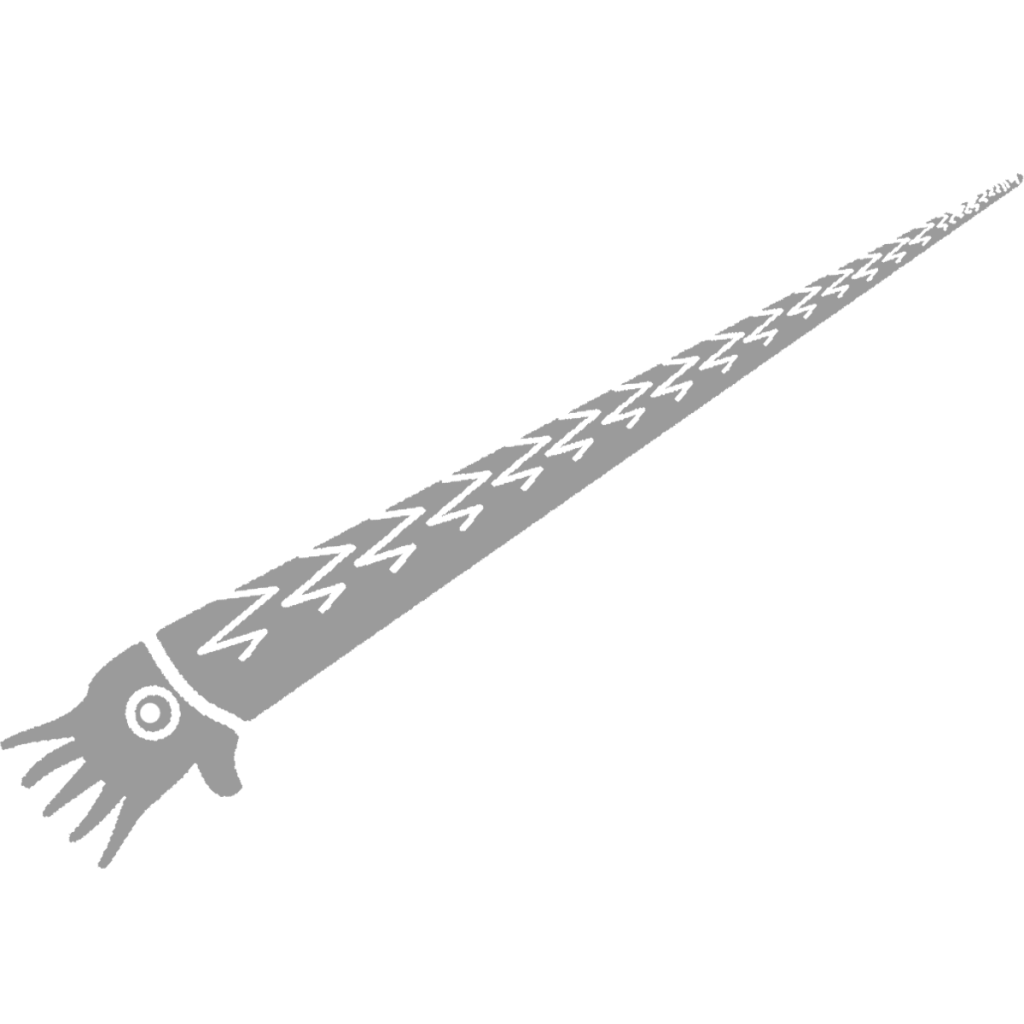
Michelinoceras telamon
RARE
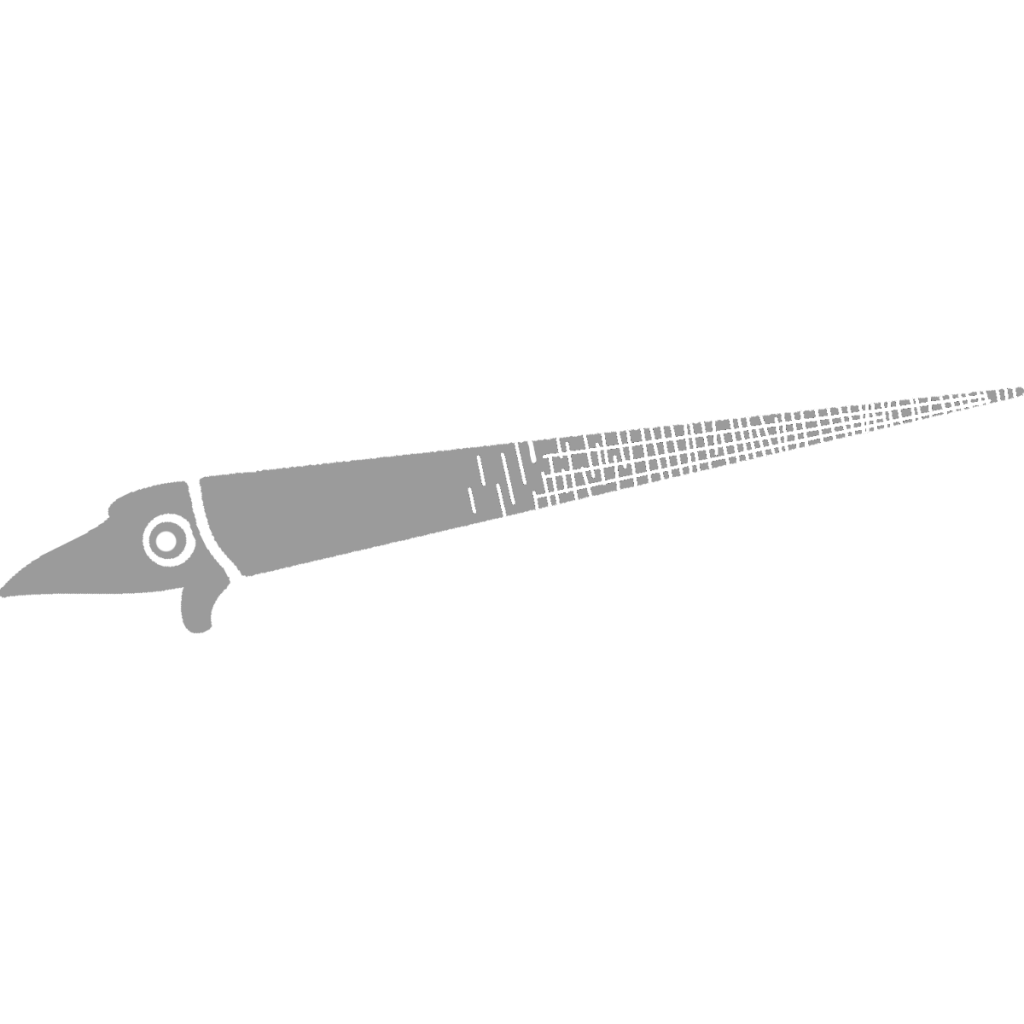
Striacoceras typum
RARE
Tornoceras uniangulare
RARE
How Do I Identify Cephalopods?
Cephalopods had chambered shells that often were filled with sediment after death. After fossilization, the original shells were either replaced by another mineral or simply deteriorated, leaving hardened casts of the chambers behind. This is especially the case for the larger cephalopods at Seven Stars. Smooth, black shells are tell-tale signs of smaller cephalopods, as long as they are the right shape – some internal molds of Ptomatis gastropods can be confused with ammonoids, but coil differently (see below).
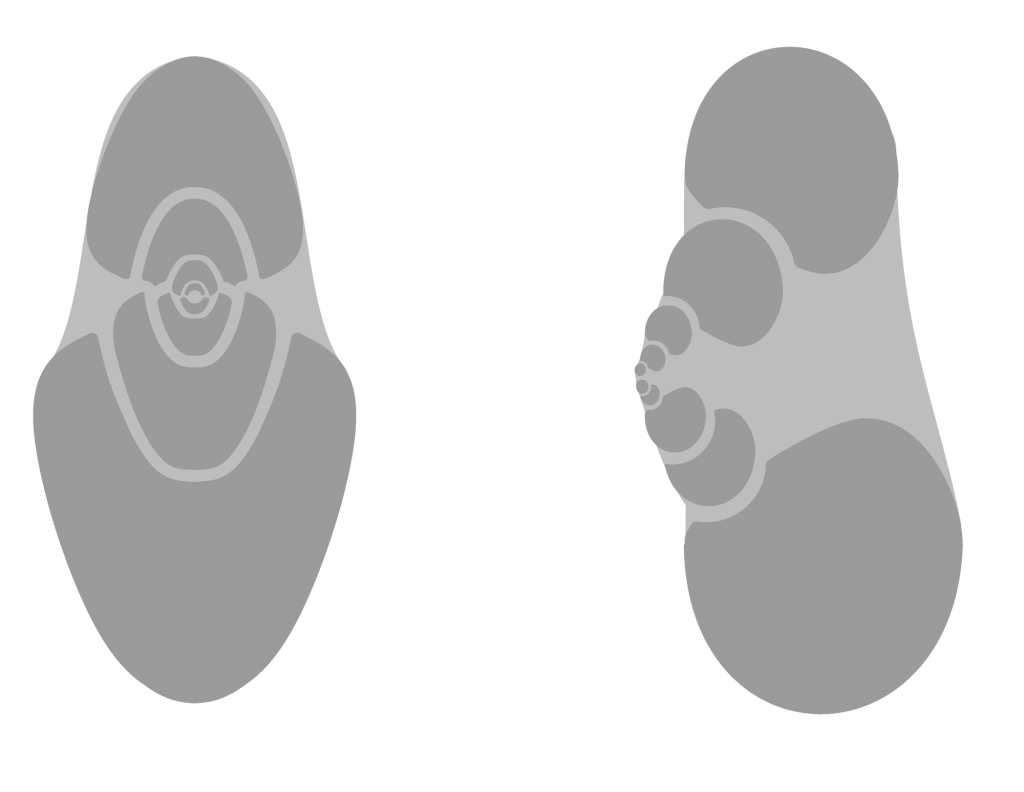
Gastropod coiling (right).
What were Cephalopods?
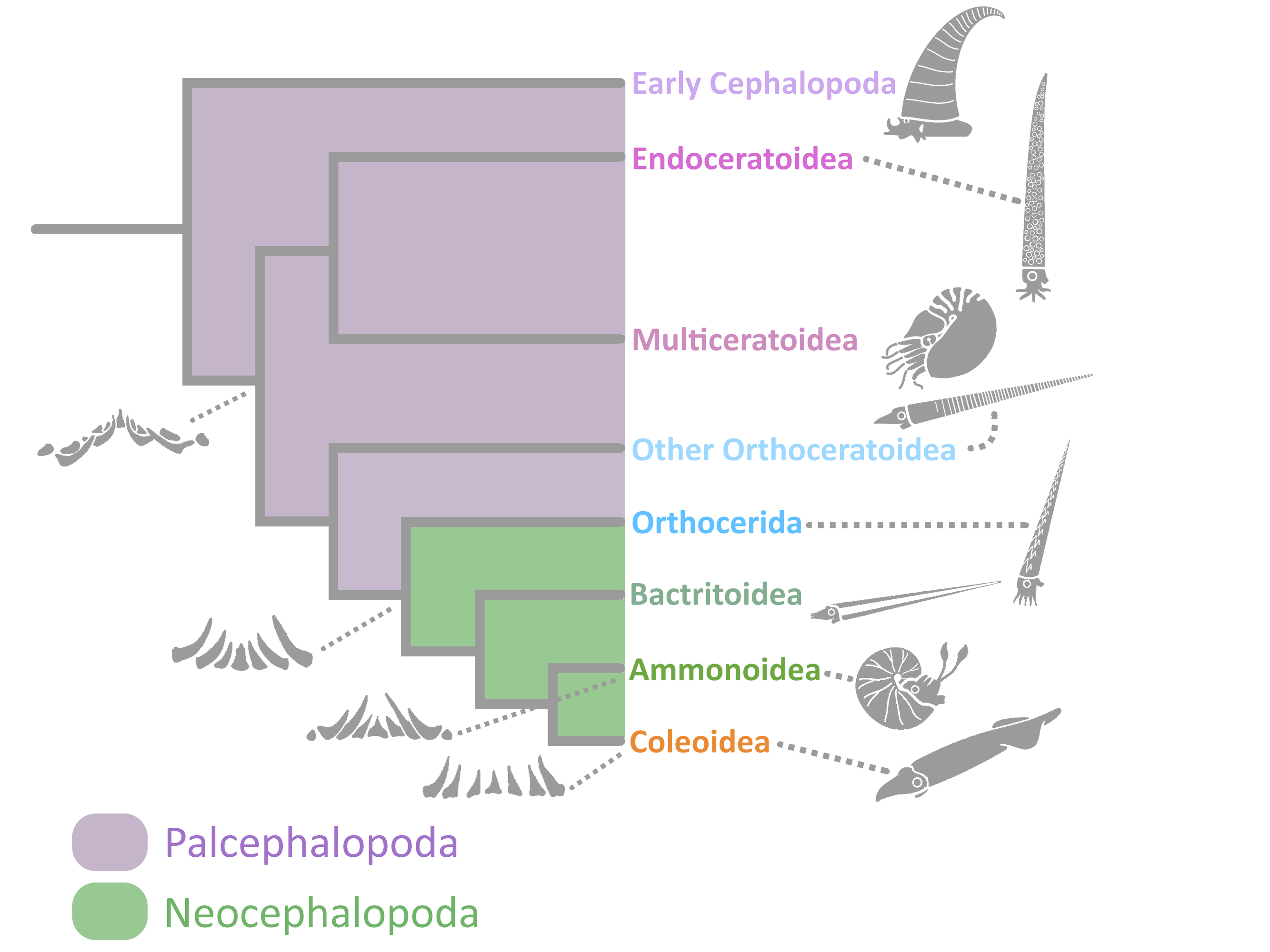
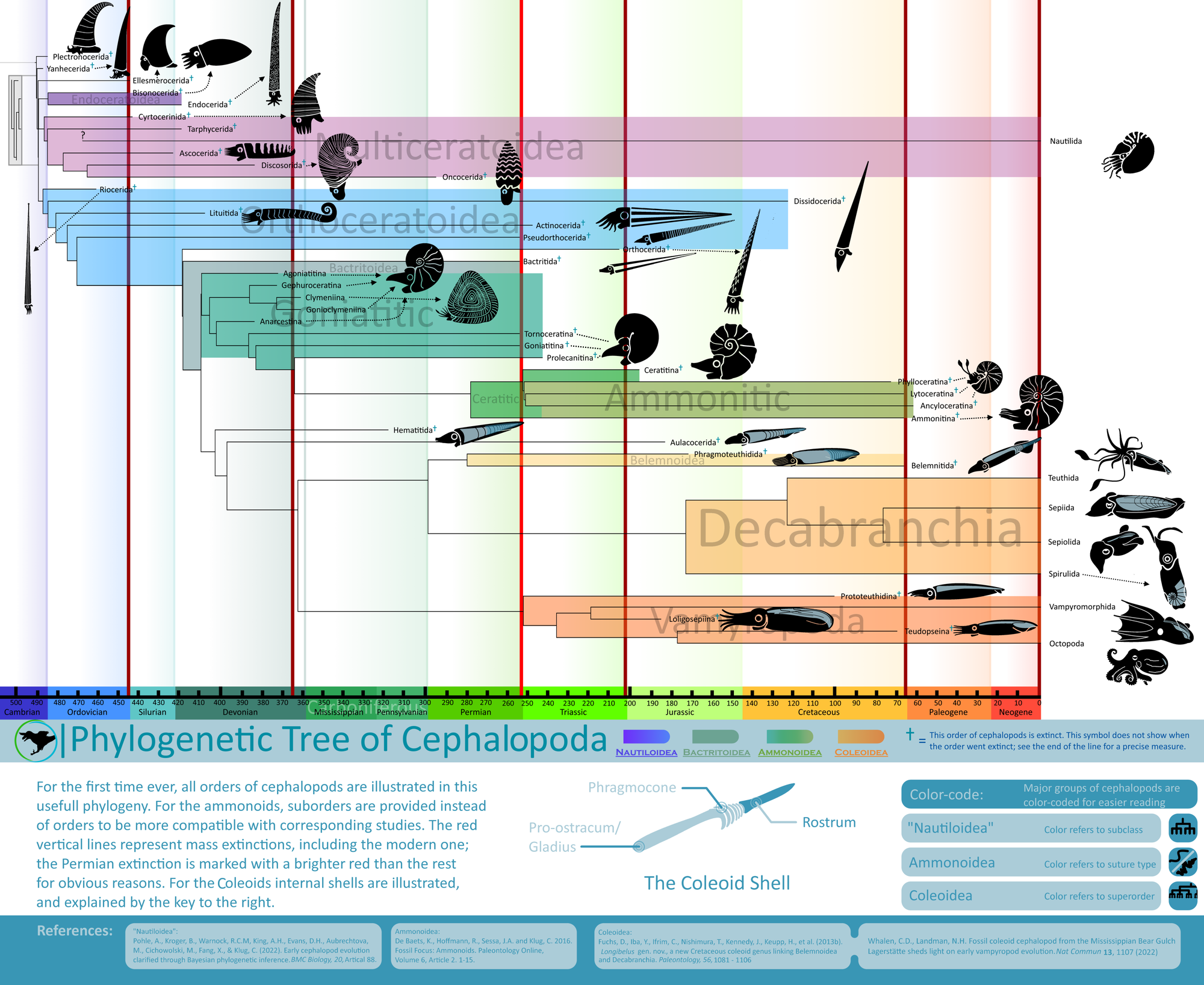
Cephalopods were mollusks. Like most mollusks, they had a tooth-covered, tongue-like organ called the radula. The number and configuration of the radular teeth differentiate the two main groups of cephalopods – the Palcephalopods and the Neocephalopods (8).
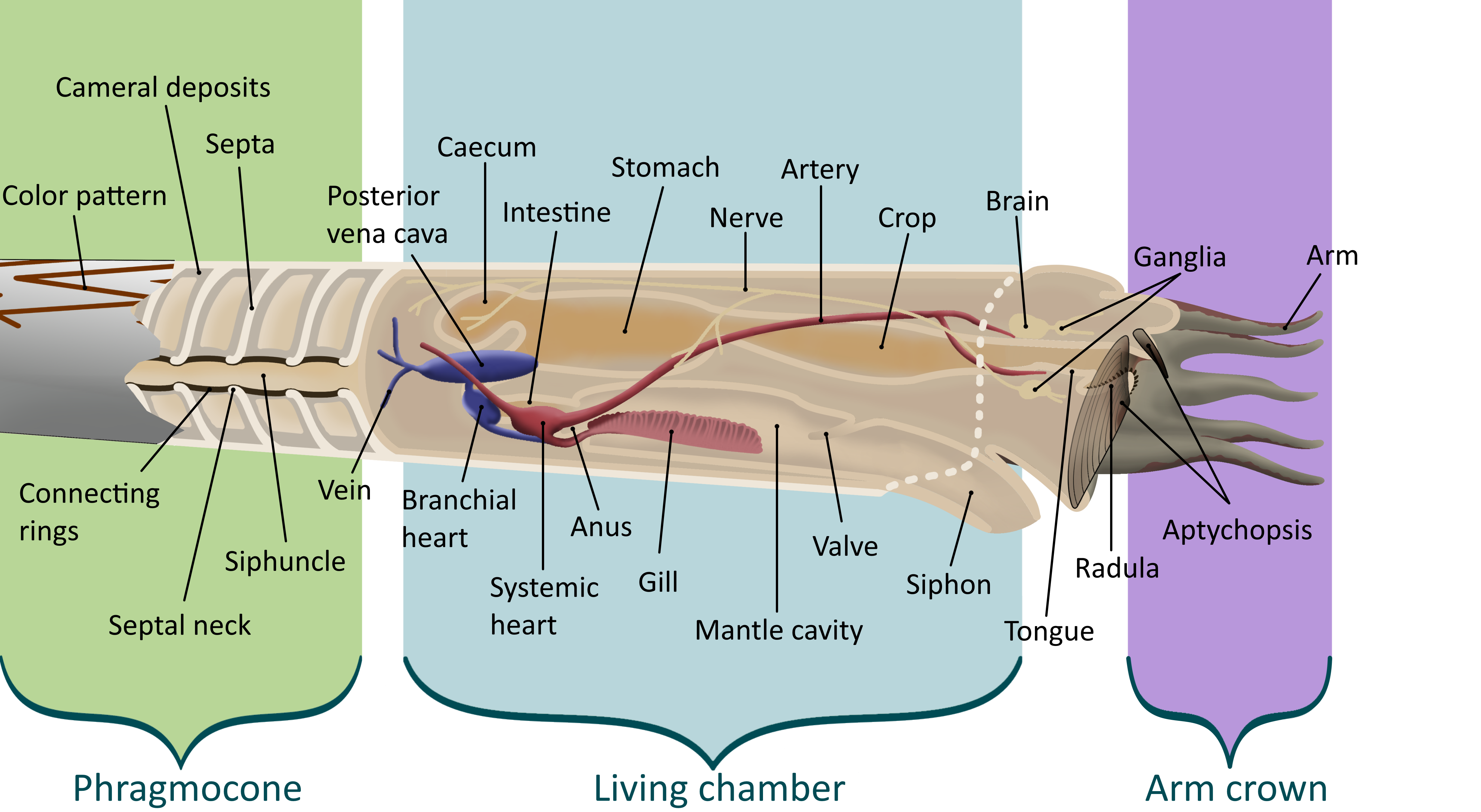
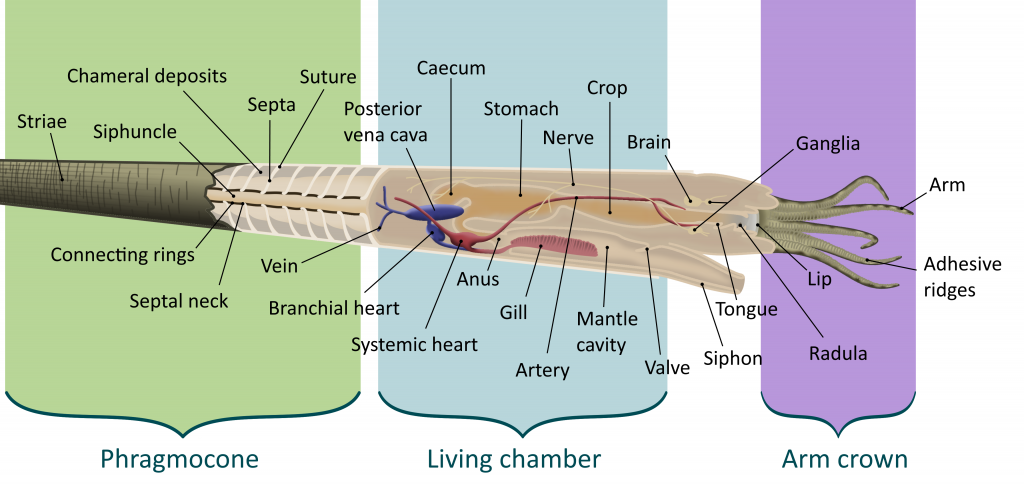
Most cephalopods were characterized by their unique shell, called a phragmocone. As the cephalopod grew, it would deposit a wall, called a septum, inside its shell, with a round hole in it. By growing many septa the cephalopod created chambers inside its shell, each filled with gas. A soft tube called the siphuncle ran through the hole in each septum, pumping fluid in and out of each chamber. By doing this, cephalopods could alter their buoyancy, letting them effortlessly rise and fall in the water column. This gave them a great advantage over other animals that had to continuously swim to maintain their position in the water column. The largest chamber, nearest the aperture (or opening) of the shell, was by far the largest and housed the living animal. While the shell is usually the only part of the cephalopod preserved, muscle attachments and other clues can help paleontologists to flesh out the soft body. This is usually as follows:
The cephalopod’s head houses the central nervous system, the eyes, mouth, and the arm crown. Cephalopods have by far the most sophisticated nervous systems of any other mollusks, helping modern octopuses to solve puzzles and escape traps in laboratory experiments (9). Unlike vertebrates, in which the eye forms from an extension of the nervous system, cephalopod eyes form from a thickening of the skin (10). As the cephalopod develops, the eye takes on a form very similar to vertebrate eyes, though cephalopods cannot see in color. Modern cephalopods may use chromatic aberration, or the refraction of different colors into different regions of the eye, due to the prismatic properties of the lense. This would let them experience color differently from many other organisms. Cephalopods would use blur and focus as a way to sense differences in how light reacts with different objects(7).
In the center of a cephalopod’s head is the mouth. In Neocephalopods, this is covered in a hard, beak-like, chitonous structure for protection and food processing. In the early Silurian, some Orthoceratoids started to protect the soft tissues around their mouths by growing chitonous plates over them (11). By the Middle to Late Silurian, these were covered in calcium, and by the Early Devonian, Bactritids and Ammonoids had lost the protective use to optimize their increasingly beak-like structures for the sole purpose of food processing. Orthocerids had especially large plates, called Aptychopsis, that effectively covered the aperture. This meant that prey with hard spines like Greenops trilobites could be more safely ingested.

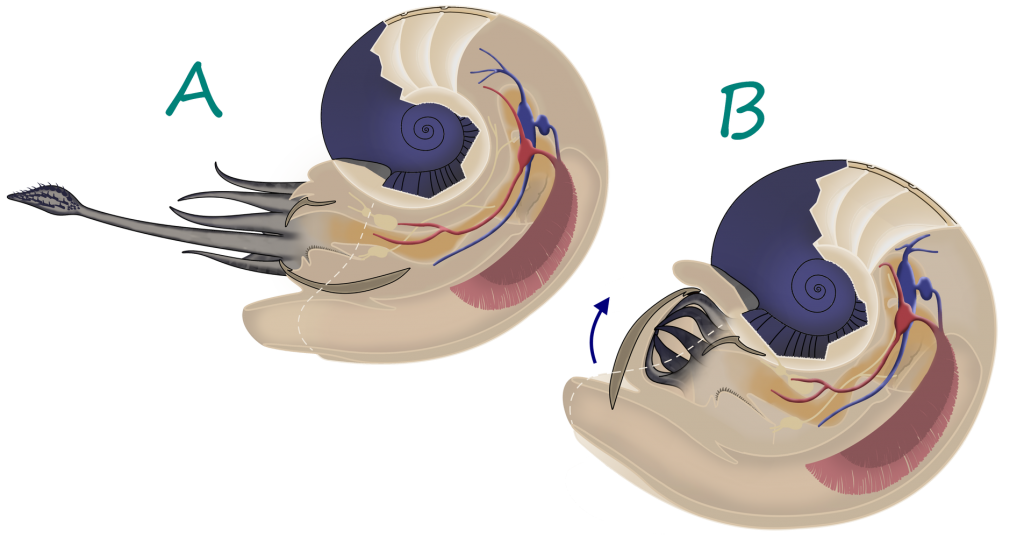
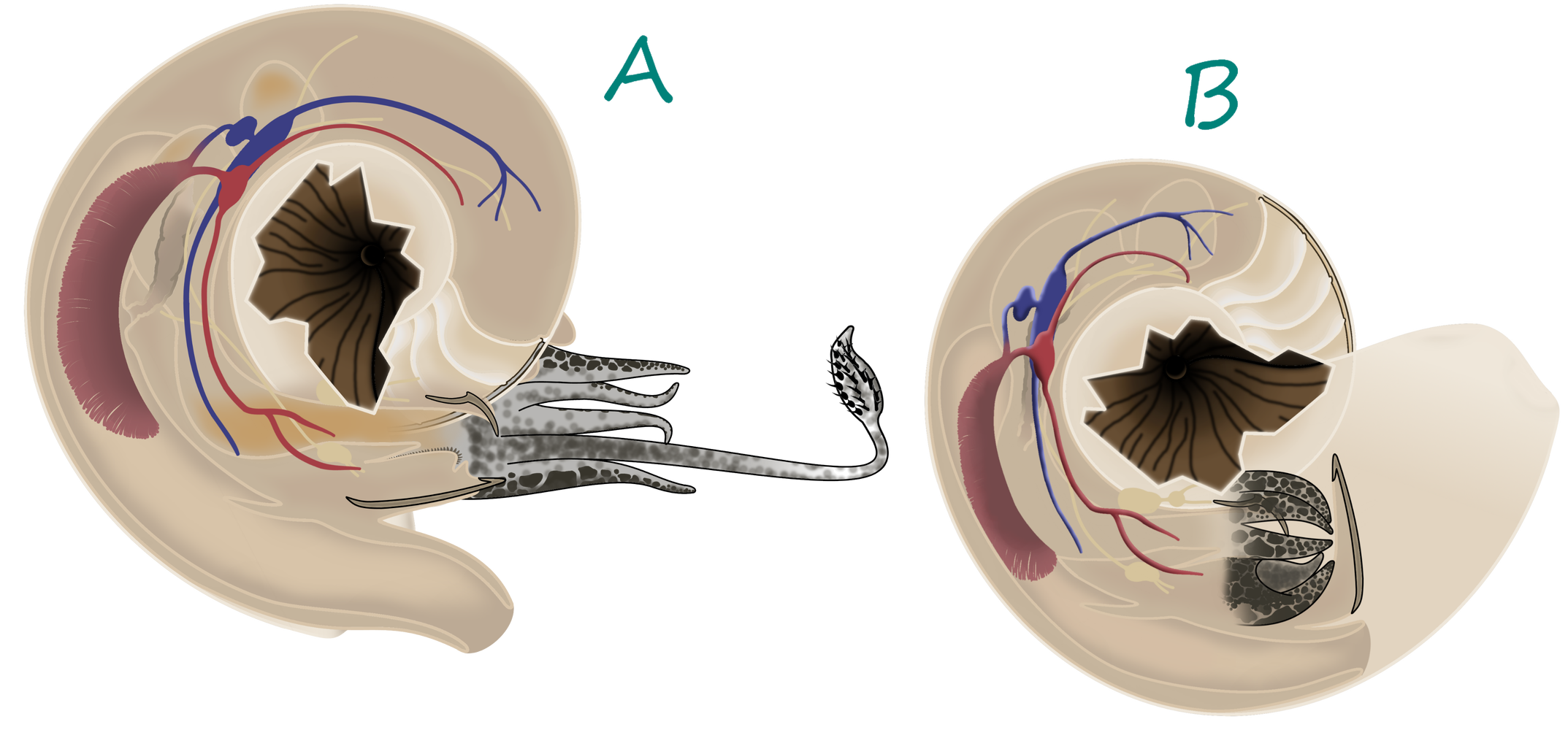
Surrounding the mouth of all cephalopods was the arm crown. This was an anterior (towards the front) extension of the molluskan foot, the posterior extension having been curled into a tube called the siphon or hyponome (2). The number and type of arms in the arm crown varies from cephalopod to cephalopod; modern nautiluses have up to 90 retractable finger-like cirri, while Orthoceratoids had 10 arms; some Cretaceous Ammonites are known to have had two elongated tentacles with hooks on the ends, similar to those of squid and cuttlefish, as well (12). The arms were used for prey capture and in some examples transportation.
The hyponome is a muscular tube used in jet propulsion, where the cephalopod pulls water into its mantle chamber and pushes it out again to produce a jet of water. Cephalopods could not swim far, because this propulsion method took a lot of energy. However, the cephalopod can accelerate much more quickly than a fish (13). This is an example of the long “arms race” between fish and cephalopods through time; once the first jawed fish appeared, as mentioned above, Orthoceratoids started to grow chitonous plates around their mouths (11).
The muscles used to contract the body chamber for jet propulsion and other actions were anchored to the shell via what is called the annular elevation, a raised portion of the posterior (towards the back) part of the living chamber. Larger annular elevations indicate larger muscle attachments and can be used to reconstruct the level of activity of ancient cephalopods (14).
Cephalopods at Seven Stars
Cephalopods, while not common when compared to Brachiopods and Bivalves, are among the most prized fossils at Seven Stars. Four genera lived at this site, all occupying slightly different ecological niches. Their presence greatly determined what the rest of the ecosystem looked like, discouraging trilobites like Eldredgeops, and favoring spiny or thick-shelled trilobites like Greenops and Dipleura.

Agoniatites vanuxemi
Although Agoniatites vanuxemi can grow up to 8 inches (20 cm) in diameter at other sites (20), it stays very small at Seven stars at only a couple centimeters max. It is easily differentiated from Tornoceras by its umbilicus, the previous whorls of the shell still visible because the new shell does not cover all of them. Agoniaties was in life more laterally compressed than Tornoceras, evidence that while it was more stable in the water column, it could not turn as easily. As an Agoniatite, it had simple sutures, making depth control less efficient than Tornoceras, which had more complex sutures (15).

Bactrities aciculum
Bactrites was a Bactritid, the group of cephalopods that arose from Orthocerids and gave rise to Ammonoids and Coleoids (the group that includes octopus, squid, etc.). Bactritids had larger muscle attachments than both Orthocerids and Dissidocerids, hinting at stronger jet propulsion and a more active lifestyle. The defining characteristic of Bactritids, however, is the ventral siphuncle (like many ammonoids) but simple sutures (like Orthocerids). While a ventral siphuncle made it much easier to pump fluid in and out of the earlier chambers (and therefore switch from a vertical to a horizontal shell orientation), it weakened the structure and made Bactritids more susceptible to crushing. Bactrites at Seven Stars and other Mahantango sites are almost crushed flat, except for rare occasions where the shell was preserved vertically. They are pyritized and have fine striae (growth lines) that differentiate them from Hyolithids and other similarly shelled organisms.

Michelinoceras telamon
Michelinoceras was an Orthocerid, having an almost central siphuncle and less closely spaced septa (20) compared to the almost completely central siphuncle and very closely spaced septa of Striacoceras typum. Internal molds are the most common fossils of Michelinoceras, being black to dark grey in color or brilliantly pyritized. Michelinoceras could grow to moderately large sizes and preferred areas that are now grey, rough-grained claystone. Michelinoceras would have automatically reverted to an almost vertical shell orientation at rest but could assume a more horizontal orientation when swimming. Possible Michelinoceras specimens from Michigan preserve the original color patterns, a light background with darker zig-zag bands on the dorsal part of the shell, suggesting the animal usually held its shell horizontally, at least while hunting. With its Aptychopsis, Michelinoceras could attack prey with hard shells and spines without much danger and could protect itself against larger cephalopods and other predators. Muscle scars from Michelinoceras’ closest relatives show that they were likely not as active as Bactrites, having weaker jet propulsion hinting at an ancestrally planktonic lifestyle; in fact early Orthocerids held their shells vertically most of the time and have been found in more open water deposits (18).

Striacoceras typum
Striacoceras was by far the largest cephalopod from Seven Stars, with body chambers hinting at a full size of almost one and a half feet (c. 45 cm). Striacoceras was a Dissidocerid, more specifically a Geisonocerid (19), a family of cephalopods that shared many features with the Orthocerids – in fact, the family has been bumped from one Order to the other many times before settling down more recently. Dissidocerids had more highly developed cameral deposits than the Orthocerids, concentrating them in the ventral posterior (towards the bottom of back of the shell) areas, presumably to help the cephalopod assume a horizontal shell orientation more easily. Dissidocerids also had larger muscle attachments, approaching the size of the Bactritids, showing that they were more active than the Orthocerids.

Striacoceras is characterized by its unique striae (growth lines, right) that changed in pattern as the shell reached maturity; usually by this time they have diminished. There is no direct evidence so far of jaws or plates in Dissidocerids; in fact early Orthocerids did not have them, suggesting they are a strictly Neocephalopod trait. Striacoceras first appeared in eastern New York and then traveled South to Pennsylvania. A very variable genus, it has 5 main variations, with examples everywhere in between; this is the only reason they are all still assigned to one genus. The example at right is variation beta; to read more about the variations read Cherry Valley Cephalopods by Rousseau H. Flower pp. 305-310, linked below.

Tornoceras uniangulare
Tornoceras was the more common ammonoid at Seven Stars and grew much larger than Agoniatites at this site, approaching its maximum size of 2 inches (5cm) in diameter (20). Tornoceras had little to no umbilicus, recent whorls almost completely covering previous ones. this helped to make the shell more compact and easier for the ammonoid to maneuver; Tornoceras had a wider shell than Agoniatites, making it more agile. As a Goniatite, with its more complex sutures (15), it could more efficiently alter its buoyancy than Agoniatites. Overall Tornoceras was a more agile ammonoid, hinting at a more active lifestyle.
Works cited
(1) Pohle, A., Kröger, B., Warnock, R.C.M. et al. Early cephalopod evolution clarified through Bayesian phylogenetic inference. BMC Biol 20, 88 (2022). https://doi.org/10.1186/s12915-022-01284-5 https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-022-01284-5
(2) Lee, Patricia & Callaerts, Patrick & de Couet, Heinz & Martindale, Mark. (2003). Cephalopod Hox genes and the origin of morphological novelties. Nature. 424. 1061-5. 10.1038/nature01872. https://www.researchgate.net/publication/10592171_Cephalopod_Hox_genes_and_the_origin_of_morphological_novelties
(3) Peterman DJ, Ritterbush KA. Resurrecting extinct cephalopods with biomimetic robots to explore hydrodynamic stability, maneuverability, and physical constraints on life habits. Sci Rep. 2022 Jul 4;12(1):11287. doi: 10.1038/s41598-022-13006-6. PMID: 35787639; PMCID: PMC9253093. https://pubmed.ncbi.nlm.nih.gov/35787639/
(4) Fuchs, Dirk & Hoffmann, René & Klug, Christian. (2021). Evolutionary development of the cephalopod arm armature: a review. Swiss Journal of Palaeontology. 140. 18. 10.1186/s13358-021-00241-z. https://www.researchgate.net/publication/357183783_Evolutionary_development_of_the_cephalopod_arm_armature_a_review
(5) Klug, C. 2007. Sublethal injuries in Early Devonian cephalopod shells from Morocco. Acta Palaeontologica Polonica 52
(4): 749–759. https://www.app.pan.pl/archive/published/app52/app52-749.pdf
(6) Flower, Rousseau H. “Cherry Valley Cephalopods.” Bulletins of American Paleontology, vol. 22, 76, 1936, pp. 273–366. Biodiversity Heritage Library, www.biodiversitylibrary.org/page/30374176#page/317/mode/1up.
(7) A.L. Stubbs, C.W. Stubbs, Spectral discrimination in color blind animals via chromatic aberration and pupil shape, Proc. Natl. Acad. Sci. U.S.A. 113 (29) 8206-8211,https://doi.org/10.1073/pnas.1524578113 (2016).
https://www.pnas.org/doi/10.1073/pnas.1524578113
(8) Tanabe, Kazushige & Fukuda, Yoshio. (1999). Morphology and function of cephalopod buccal mass. https://www.researchgate.net/publication/256077353_Morphology_and_function_of_cephalopod_buccal_mass
(9) Asada, Keishu & Nakajima, Ryuta & Nishibayashi, Takahiro & Ziadi-Künzli, Fabienne & Lajbner, Zdeněk & Miller, Jonathan & Gutnick, Tamar & Kuba, Michael. (2021). Improving Keeping for Octopuses by Testing Different Escape- Proof Designs on Tanks for “Big Blue Octopus” (Octopus cyanea). Journal of Applied Sciences. 11. 10.3390/app11188547. https://www.researchgate.net/publication/354586499_Improving_Keeping_for_Octopuses_by_Testing_Different_Escape-_Proof_Designs_on_Tanks_for_Big_Blue_Octopus_Octopus_cyanea
(10) William, Harris. (1997). Pax-6: Where to be conserved is not conservative. Proceedings of the National Academy of Sciences of the United States of America. 94. 2098-100. 10.1073/pnas.94.6.2098. https://www.researchgate.net/publication/14096479_Pax-6_Where_to_be_conserved_is_not_conservative
(11) Mironenko, Aleksandr. (2021). Early Palaeozoic Discinocarina: a key to the appearance of cephalopod jaws. Lethaia. 10.1111/let.12414. https://www.researchgate.net/publication/347354135_Early_Palaeozoic_Discinocarina_a_key_to_the_appearance_of_cephalopod_jaws
(12) Smith, C.P.A., Landman, N.H., Bardin, J. et al. New evidence from exceptionally “well-preserved” specimens sheds light on the structure of the ammonite brachial crown. Sci Rep 11, 11862 (2021). https://doi.org/10.1038/s41598-021-89998-4 https://www.nature.com/articles/s41598-021-89998-4?fbclid=IwAR1MjmlTjxXHUWRSlCrxgaFTylhnzsRM4Q_DjcHLX96eaj_xpGnQ04efefQ
(13) Wells, Martin J.; O’Dor, R. K. (July 1991). “Jet Propulsion and the Evolution of the Cephalopods”. Bulletin of Marine Science. 49 (1): 419–432(14). https://www.ingentaconnect.com/content/umrsmas/bullmar/1991/00000049/f0020001/art00038
(14) Kröger, B., Klug, C., and Mapes, R. 2005. Soft−tissue attachments in orthocerid and bactritid cephalopods from the Early and Middle Devonian of Germany and Morocco. Acta Palaeontologica Polonica 50 (2): 329–342. https://www.academia.edu/588647/Soft_tissue_attachments_in_orthocerid_and_bactritid_cephalopods_from_the_Early_and_Middle_Devonian_of_Germany_and_Morocco
(15) Korn, Dieter & Klug, Christian. (2002). Korn & Klug 2002 Ammoneae Devonicae chapter 3 AGONIATITIDA. https://www.researchgate.net/publication/264349111_Korn_Klug_2002_Ammoneae_Devonicae_chapter_3_AGONIATITIDA
(16) Mawson, Ruth & Talent, John. (2003). Conodont faunas from sequences on or marginal to the Anakie Inlier (Central Queensland, Australia) in relation to Devonian transgressions. Bulletin of Geosciences. 78. https://www.researchgate.net/publication/228599964_Conodont_faunas_from_sequences_on_or_marginal_to_the_Anakie_Inlier_Central_Queensland_Australia_in_relation_to_Devonian_transgressions
(17) Peterman, David J., Barton, Christopher C., and Yacobucci, Margaret M. 2019. The hydrostatics of Paleozoic ectocochleate cephalopods (Nautiloidea and Endoceratoidea) with implications for modes of life and early colonization of the pelagic zone.
Palaeontologia Electronica 22.2.24A 1-29. https://doi.org/10.26879/884
palaeo-electronica.org/content/2019/2521-cephalopod-hydrostatics
(18) Kröger B, Servais T, Zhang Y. The origin and initial rise of pelagic cephalopods in the Ordovician. PLoS One. 2009 Sep 30;4(9):e7262. doi: 10.1371/journal.pone.0007262. PMID: 19789709; PMCID: PMC2749442. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2749442/
(19) Uhen, M.D., B. Allen, N. Behboudi, M. E. Clapham, E. Dunne, A. Hendy, P. A. Holroyd, M. Hopkins, P. Mannion, P. Novack-Gottshall, C. Pimiento, and P. Wagner. 2023. Paleobiology Database User Guide Version 1.0. PaleoBios 40(11): 1-56.
DOI: https://doi.org/10.5070/P9401160531. https://paleobiodb.org/#/.
(20) Wilson, K. A. (2014). Field guide to the Devonian fossils of New York (3rd ed.). Paleontological Research Institution.
(21) Flower, R.H. (1955b). Trails and tentacular impressions of orthoconic cephalopods. Journal of Paleontology, 29(5),857-867. https://sci-hub.se/https://www.jstor.org/stable/1300406?seq=1
