Conodonts are perhaps the most important fossils to our daily life because of their use as index fossils. Their “teeth,” called conodont elements, preserve well and can be found in almost any Paleozoic sedimentary rock. While traditionally seen as vertebrates, even fish, their taxonomic positions are still disputed (4).
Conodonts found at Seven Stars
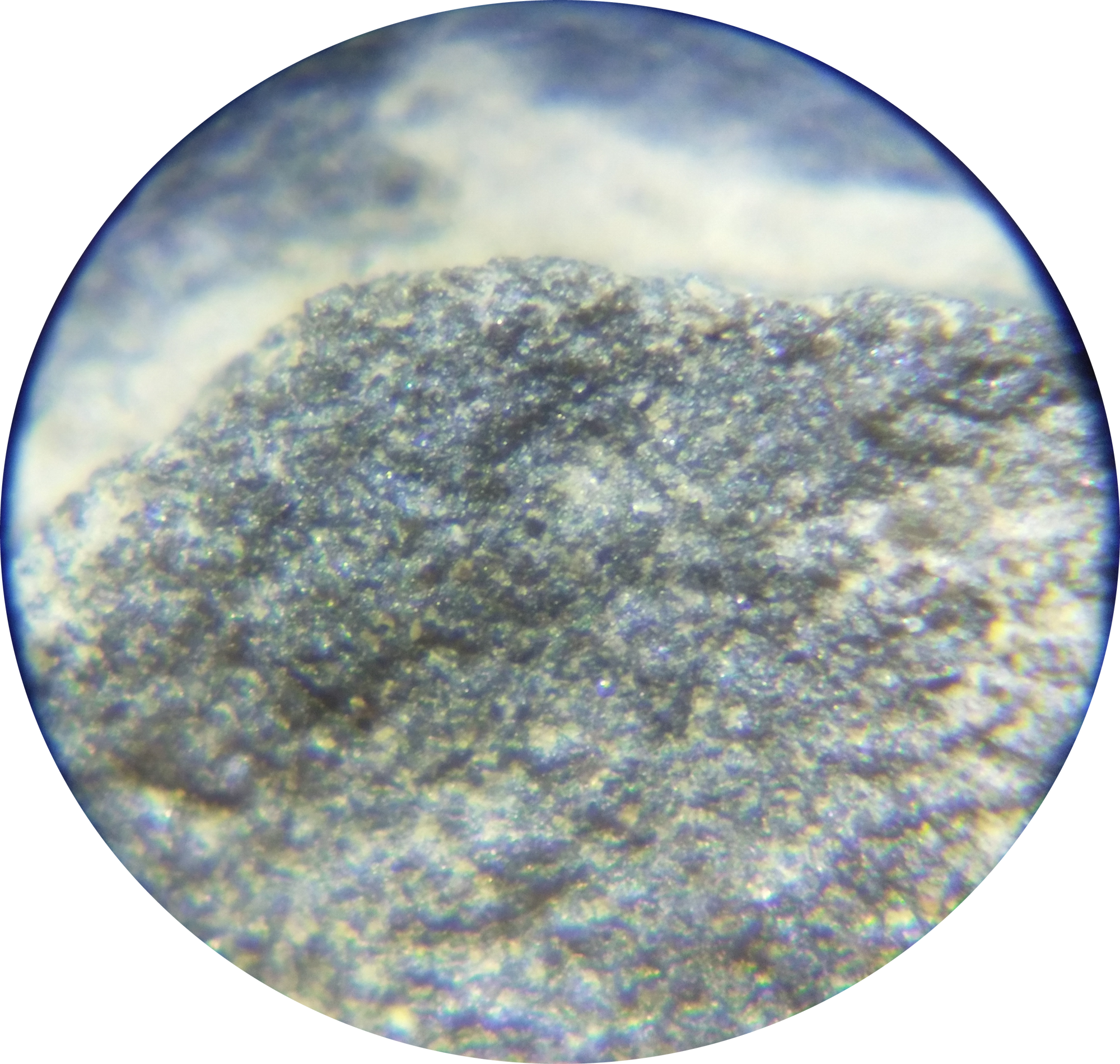
Conodonta indet.
COMMON
How do I identify Conodonts?
Conodonts are very hard to document at Seven Stars because of their preservation and the rock around them. In other shales that are high in organic matter, conodonts can be extracted effectively from the rock using acids such as Hydrogen peroxide, because they oxidize the carbon in the rock. This breaks the rock down to a residue that can be sifted through or searched with a microscope for conodont elements. However, Seven Stars is part of the Mahantango Formation, which is especially low in organic matter. When Seven Stars rocks are bathed in Hydrogen peroxide, they will bubble, but will not do much but clean the rock. Therefore it is difficult to study conodonts at Seven Stars; observation is reduced to searching the surface of a rock with a microscope at roughly 60x magnification. Even this is not satisfactory; while vague shapes and denticles can sometimes be seen, all are partially covered in matrix, are broken, or are fused together with other elements, making identification almost impossible.
Conodont elements, despite their bad preservation, are innumerable at Seven Stars. While many elements would have belonged to the same animal, many animals would have been necessary to produce the large amount of conodont elements that can be seen in Seven Stars rocks.
It is common that many kinds of conodonts lived in the same ecosystem; chemical studies have concluded that they occupied different niches and fed on different food sources to avoid competition (18).
What were Conodonts?
It is important to note the difference between how the tooth-like fossils called conodonts and the animal they belonged to are referred. Usually, the oral structures are called Conodont elements, and the entire animal is called a conodont. Controversies arise when the term “Conodont animal” is used, traditionally because one would not refer to a “Brachiopod animal”.
Conodont elements were mostly microscopic, phosphatic structures curiously grown from the external surface (4). This is confirmed because the imprints of cells have been noted on the external surface of some crushing elements 3. This meant that when not in use, conodont elements must have been covered in living tissue used to secrete more and more layers of phosphatic mineral. Because of this unique mechanism, conodonts could easily have repaired broken elements by secreting a new layer over a given fracture. This has been commonly observed, with broken points of elements being slightly offset, but healed over (4).
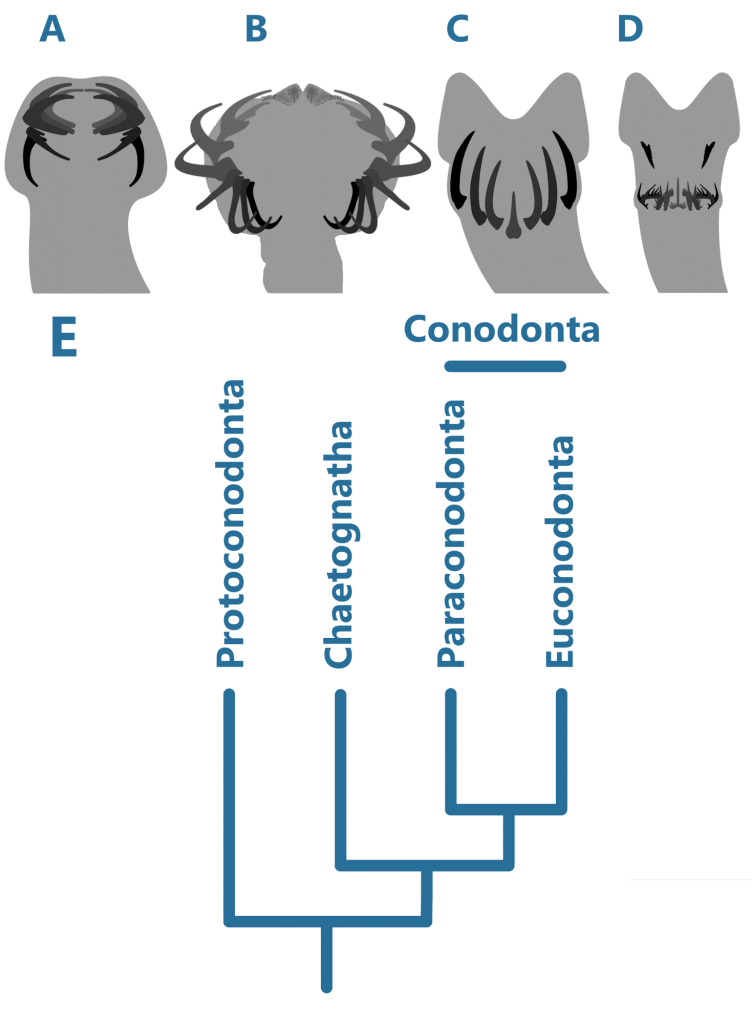
Traditionally, three major groups of conodonts have been accepted, but this view has since changed (9). In the early Cambrian Period, the Protoconodonts were the first to appear. It is then to be expected that the next group, the Paraconodonts, derived from the earlier, with a very distinct internal structure – in fact, there are no homologous tissues between these groups (2). The Euconodonta was much more diverse than the Paraconodonta, and had a more complex internal structure, discussed below.
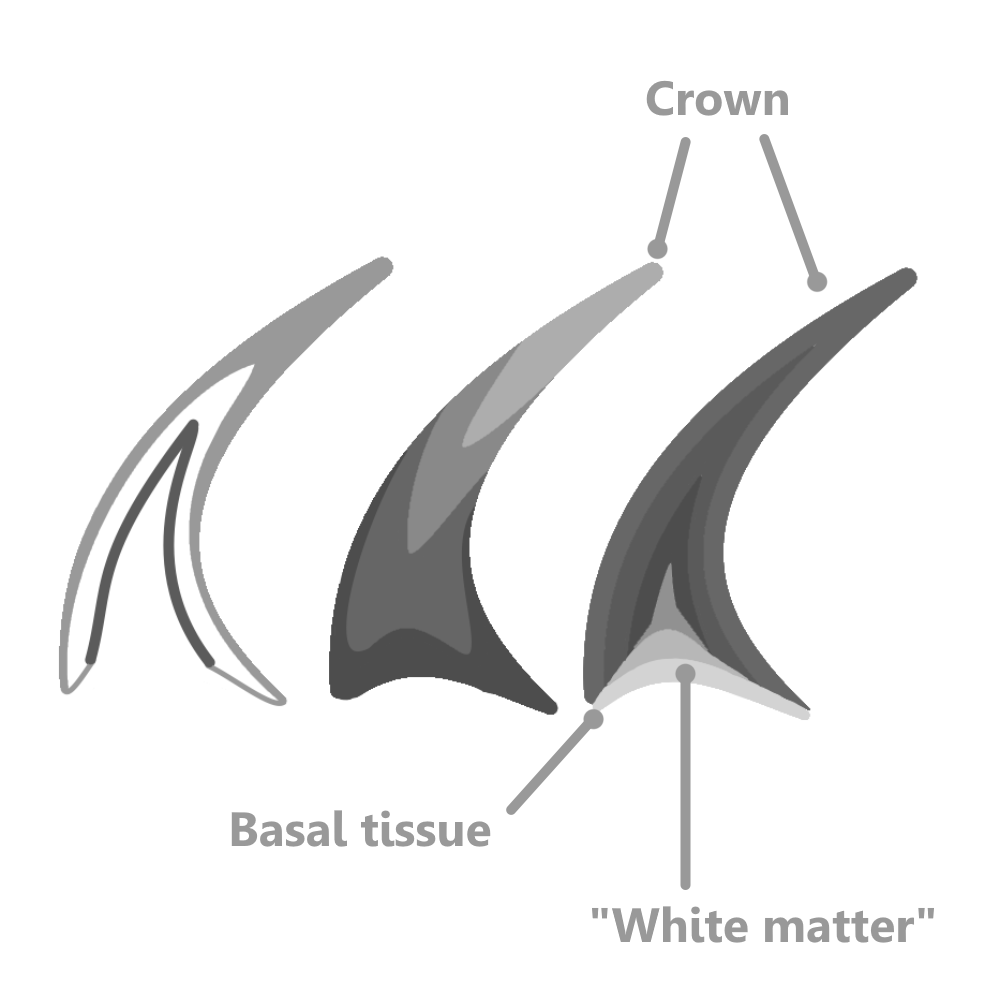
Conodont Element Anatomy
In early ontogeny, the Paraconodonts simply secreted layers onto their elements similar to stacking cups from below. As the conodont matured, a depression formed on the base of the element, known as the basal cavity 5.
In Euconodonts, a basal cavity is always present, but is mostly filled with a fibrous substance called basal tissue, made up of “white matter”. White matter was a lamellar, spongy substance with many small internal pockets, appearing white under visible light but black under other kinds. This was suggested to be similar to bone by some authors seeking to place Conodonta in Vertebrata (4). However, as pointed out by Turner et. al. (2010), the pockets inside white matter were too small to have housed osteocytes (bone cells). Similarly, because of the crystals that made up the crown of Paraconodont and Euconodont elements, some authors tried to find homologies between them and enamel, a strictly Vertebrate tissue. However, the crystals inside the crowns of conodont elements are too large and disorderly to be enamel 4.
Feeding Strategies
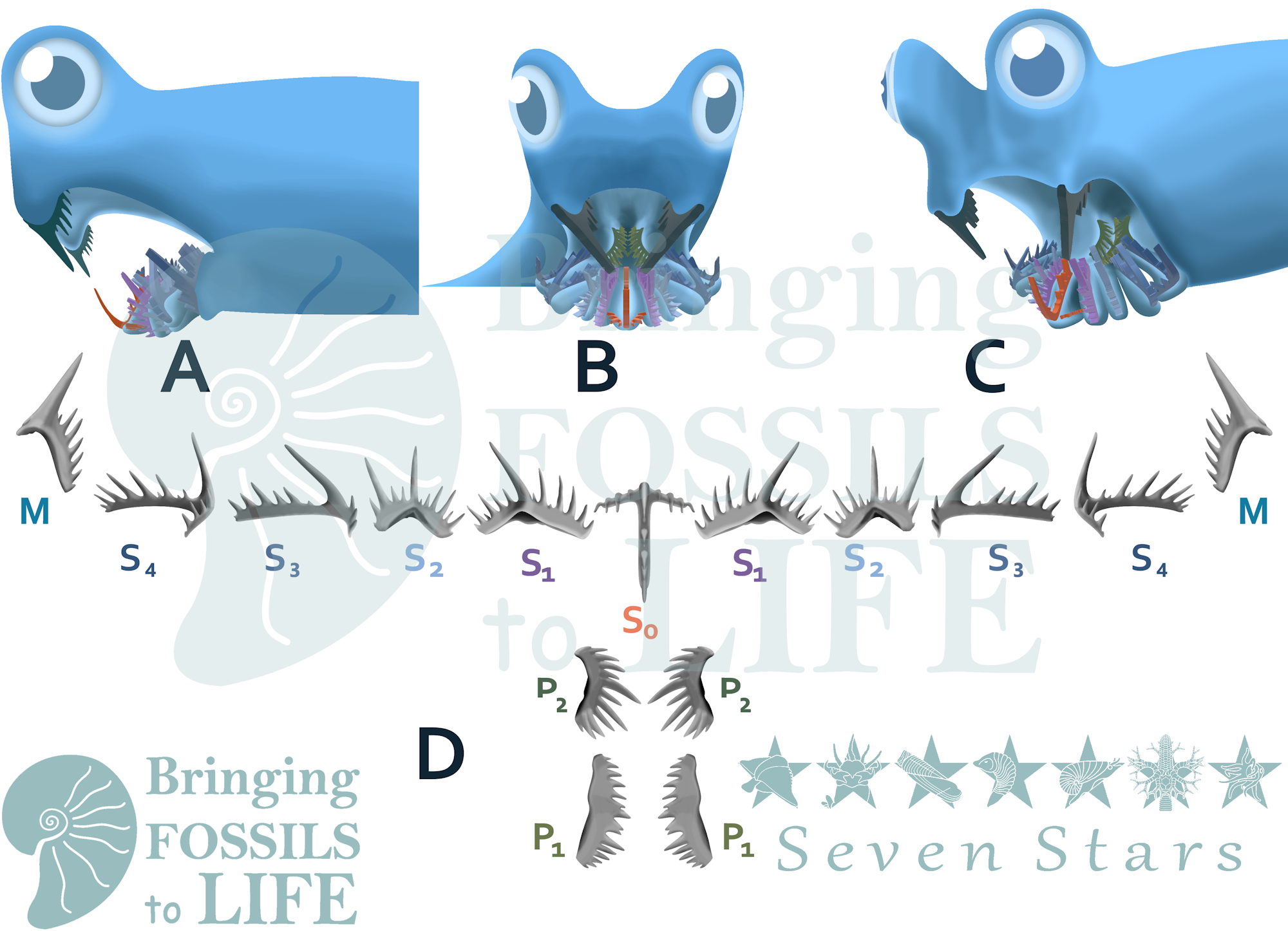
There has been major debate on the function of Conodont elements. The three main arguments are filter feeding, parasitic feeding, and grasping and crushing. The filter feeding argument consists of the elements supporting a soft, not easily preserved structure used to filter plankton out of the water (4). This satisfies the need for the conodont elements being covered by soft tissue, allowing them to grow. In the parasitic feeding argument, Conodonts have no control of the movements of their elements, simply using them to latch onto larger soft bodied organisms. The longest cusps of the conodont elements would make first contact, and the smaller denticles would rasp away at the host organism as it thrashed, trying to remove the feeding conodont (5). The grasp and crush theory entails a conodont using its anterior S elements (see illustration at end of post) to grasp smaller organisms, and using the more posterior P elements to either grind or shred the prey, depending on what kind of P elements the conodont had.
The filter feeding argument overlooks some more recent studies on the mechanical performance of conodont elements. Because Conodonts had no jaws on which to anchor muscles, they could not wield their elements with much force. Mechanical studies have concluded that they made use of all the force efficiently, by concentrating it on very small points on the prey’s body. This meant that conodont elements were exceedingly sharp (11), which does not align with the filter feeding argument. Also discouraging this view is the common wear on conodont elements that suggest occlusion and much physical use (12).
The Parasitic feeding argument looks convincing, but many conodonts had P elements that obviously had a grinding purpose. A parasitic feeder that fed on soft tissue would have little need of grinding elements; instead, shredding elements would be more beneficial. No remains of large, soft bodied organisms have been found at Seven Stars or similar environments, even in places where much soft tissue is preserved. This leaves the grasp and crush method of feeding as the most likely.
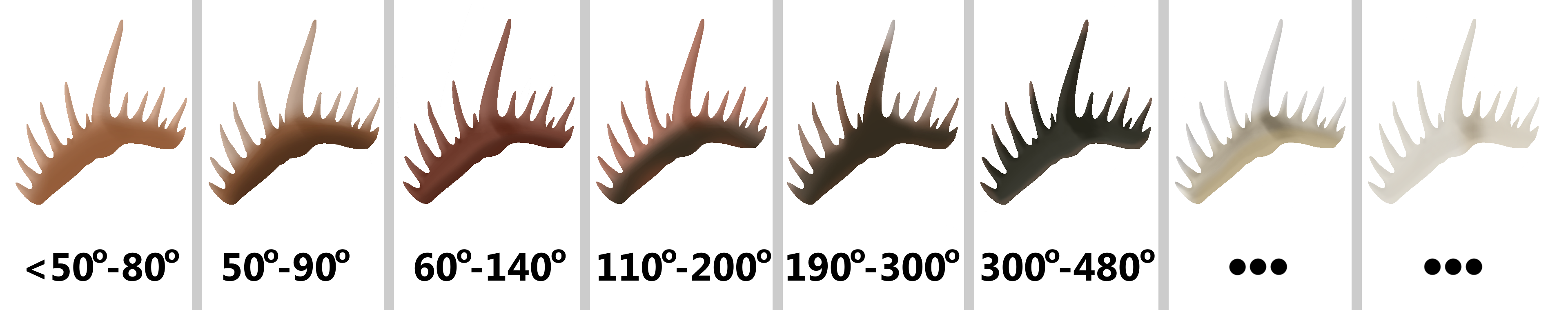
Conodont Alteration Index
Importantly to geologists, the crowns of conodont elements will change color predictably when pressure is applied to them (13). Carbon impurities in the layers of their crowns will change as a result of heat and time, and between the fourth and fifth documented levels of change, conodont elements become rough and grainy. Once considerably above 300°, all carbon will be driven out, producing white and clear elements. Since the bases of conodont elements have more organic matter in them, they change first. This was tested by heating up conodont elements that were previously thought not to have experienced much pressure, simulating the conditions that would cause them to change. A system was developed to predict how much rock overlayed a strata with conodonts of a certain color in it, called the Conodont Alteration Index (13). The darker the conodont element, the more pressure was applied to it by overlying rock. This index can be used to reconstruct how much rock was once in a place, if it was worn away be glaciers or other weathering.
Soft Anatomy
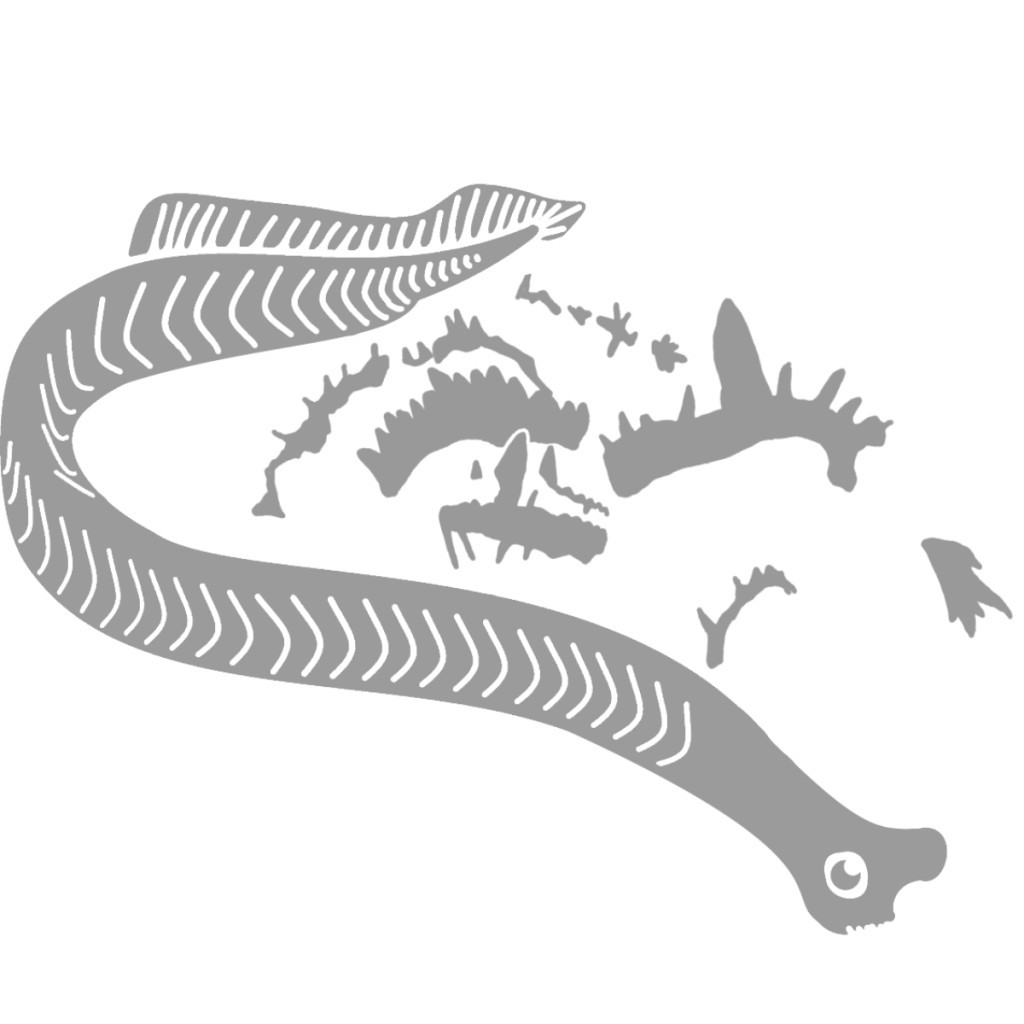
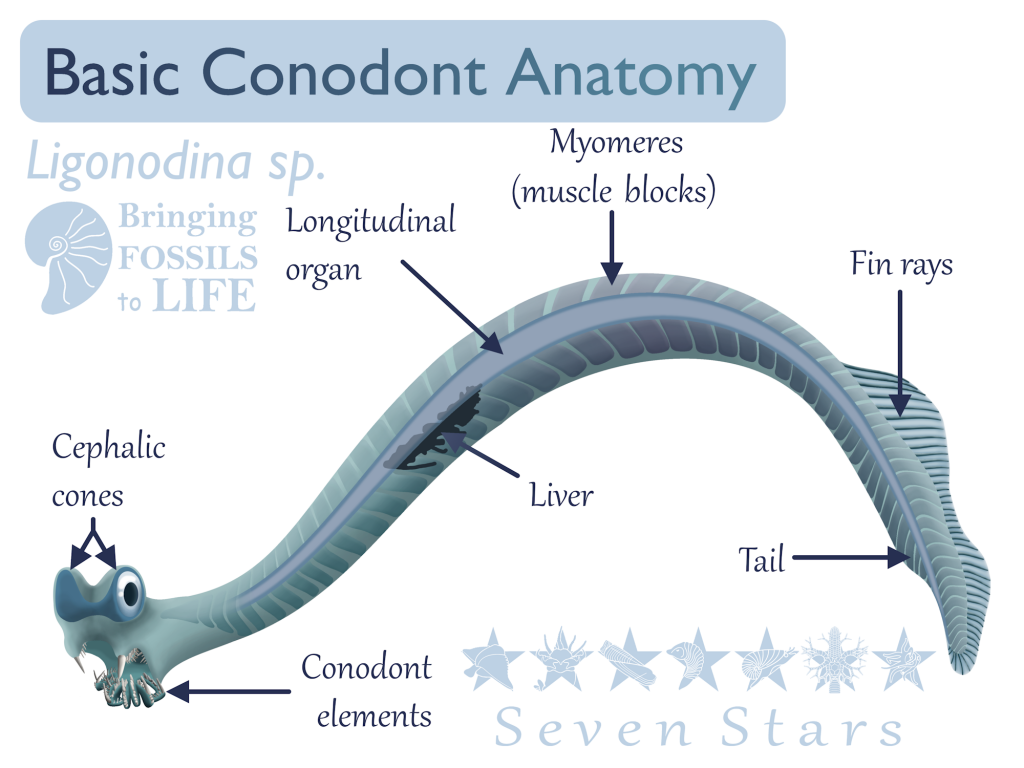
For a long time, only conodont elements were known, leaving the true nature of the organisms that bore them open to speculation. Each individual conodont element was given its own genus and species, a taxonomic approach that some still use within biostratigraphy. A possible match, called Typhloesus, was discovered, based on the presence of conodont elements inside the body. However, Typhloesus has been proposed to have been a mollusk (19), likely related to Gastropods, that actively swam in open water and fed on conodonts; the conodont elements within its body were inside its gut, not its mouth. When an exceptional specimen of an eel-like animal containing the elements of Clydagnathus was found, much progress was made concerning the taxonomic placement of the conodonts.
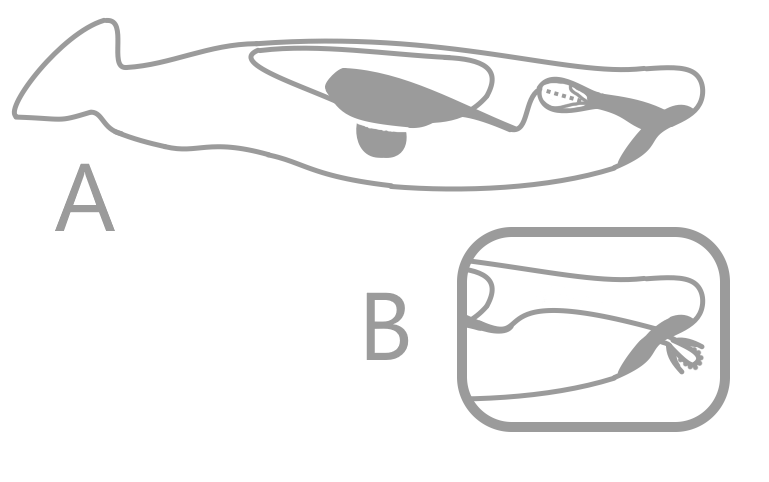
Clydagnathus had a long, thin body, a small head, and a flattened tail. V-shaped muscle blocks and a long longitudinal organ ran down the entire body, including the tail. The tail bore a dorsal, lobed fin, supported by cephalochordate-like fin rays (6). The head, which contained the conodont elements, also bore two conjoined, cone-shaped structures rimmed in muscles. While the true purpose of these cones remains unknown, they are situated perfectly to be eyes, though no other organisms have eyes with similar structure (4).
Classification
While the Clydagnathus fossil did much to answer many questions about conodonts, it also opened many more. In the original paper describing the fossil, Briggs et. al., 1983, provided three theories as to where Conodonta fit in with other animal life. The first theory was that Conodonts belonged in the phylum Chordata, the group that includes all vertebrates, clyclostomes (hagfish and lampreys), tunicates, and cephalochordates (lancelets). Chordata is characterized by the presence of a stiff, yet flexible support called the notochord, running the length of the body. The longitudinal organ found in conodonts was thought to have possibly represented a notochord, if not a gut (6).
The second theory of classification was that Conodonts were related to the phylum Chaetognatha, or arrow worms. Chaetognaths are known as common plankton today, and have multiple pectoral fins as well as a mouth fringed by long, grasping teeth. Conodonts were known to have ray-supported fins, and Chaetognaths are the only organisms to possess these other than the Chordates (6).
The third theory was that Conodonts fit into their own phylum, because they were not quite like any other organisms (6). This was the favored theory by Briggs et. al., but is not accepted by most scientists today.
Originally, the Chordate theory took off. Soon, scientists took this theory to the extreme, classifying the conodonts as vertebrates, and then even agnathans (jawless fish) – only a few authors believed otherwise (4). The conodonts as vertebrates theory was printed in dinosaur books for kids, and was unquestioned by most of the scientific community.
But still, not all experts were so thrilled by this new classification. In 2010, Turner et. al. published an extensive argument that dismissed the agnathan (jawless fish) theory, some of the main points summarized below.
Amending the Chordate Theory
Many authors had tried to homologize conodont elements with vertebrate tissues, such as bone. As mentioned above, they claimed that the lamellar crowns of conodont elements, made up of hyaline, represented enamel because of their crystal structure. However, the crowns of conodont elements were grown from the outside, unlike enamel, and were made up of larger crystals in a less organized pattern than enamel – different genera had different patterns, but none had the smaller prismatic structures found in enamel. They also claimed that white matter was bone, because it possessed gaps that in theory could have been filled with osteocytes, or bone cells. However, the open pockets within white matter were too small to have held osteocytes, meaning that they could not have been bone. While the absence of these tissues in conodonts does not mean that they could not have been vertebrates, it strips away conclusive evidence of this.
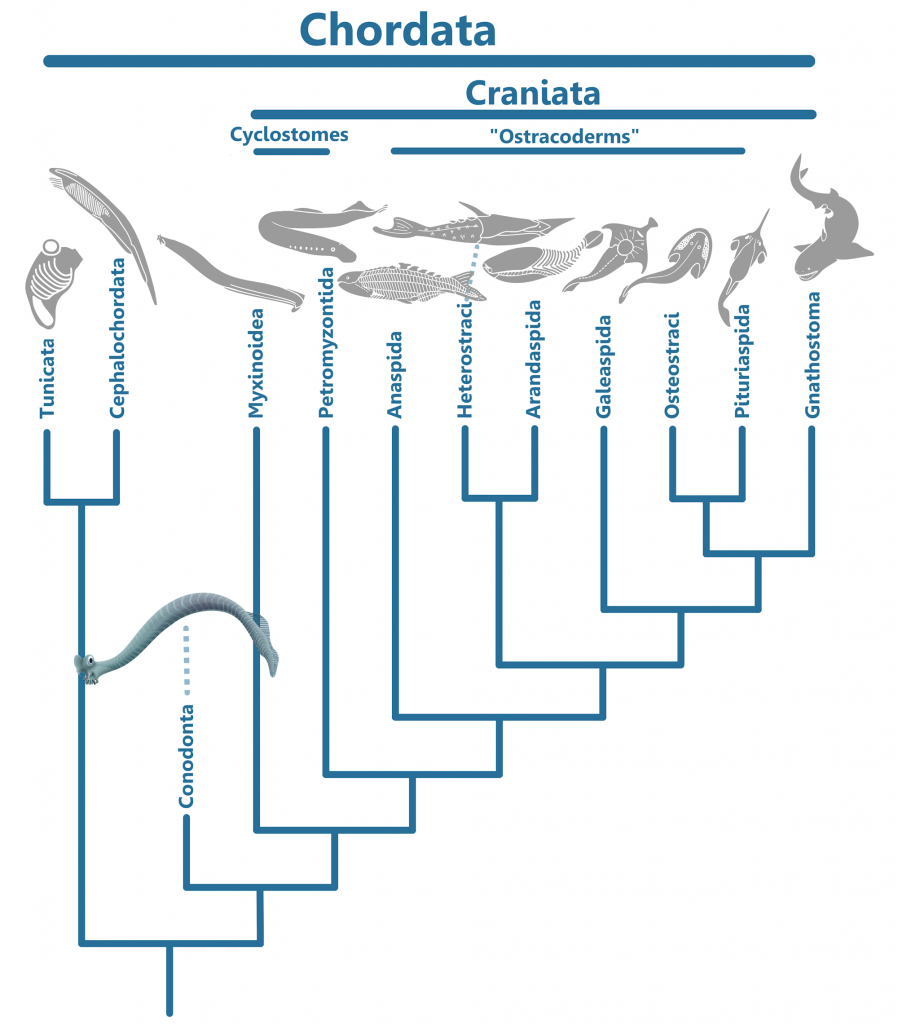
The muscle blocks, or myomeres, that conodonts possessed, were V-shaped, not the W-shape found in true vertebrates, or the larger Craniata, which also included the cyclostomes (the lampreys and hagfishes). Unlike Conodonts, the Craniata is characterized by a cranium, a hard structure protecting the central nervous system. Instead, both conodont myomeres and fin rays were more similar to those of the the Cephalochordates, which were not Craniates.
Another piece of evidence against a Craniate taxonomic placement for the Conodonta is the lack of the level of cephalization seen in the former group. In conodonts there are no known nasal organs, leaving the conodont elements and the strange, cone-shaped organs as the only confirmed cephalic structures. The cone-shaped structures are unlike any organs found in Craniates, but are in similar positions as eyes. They are usually preserved as dark stains and are also obvious in Promissum pulchrum. Some Cephalochordates possess eyespots, so it is possible that these organs were optical in function as originally speculated.
Promissum pulchrum was a comparatively huge conodont from the South African Soom Shale. Only the anterior half of the body is known, but if its proportions were similar to Clydagnathus, then it could have reached 40 centimeters in length.
From this evidence, it seems rather obvious that conodonts were neither Vertebrates nor Craniates, but that they shared similarities with Cephalochordates. Turner et. al. placed the Conodonta as sister taxa to the Craniates, being less derived than the Cyclostomes but more derived than Tunicates and Cephalochordates.
The Chaetognath Theory
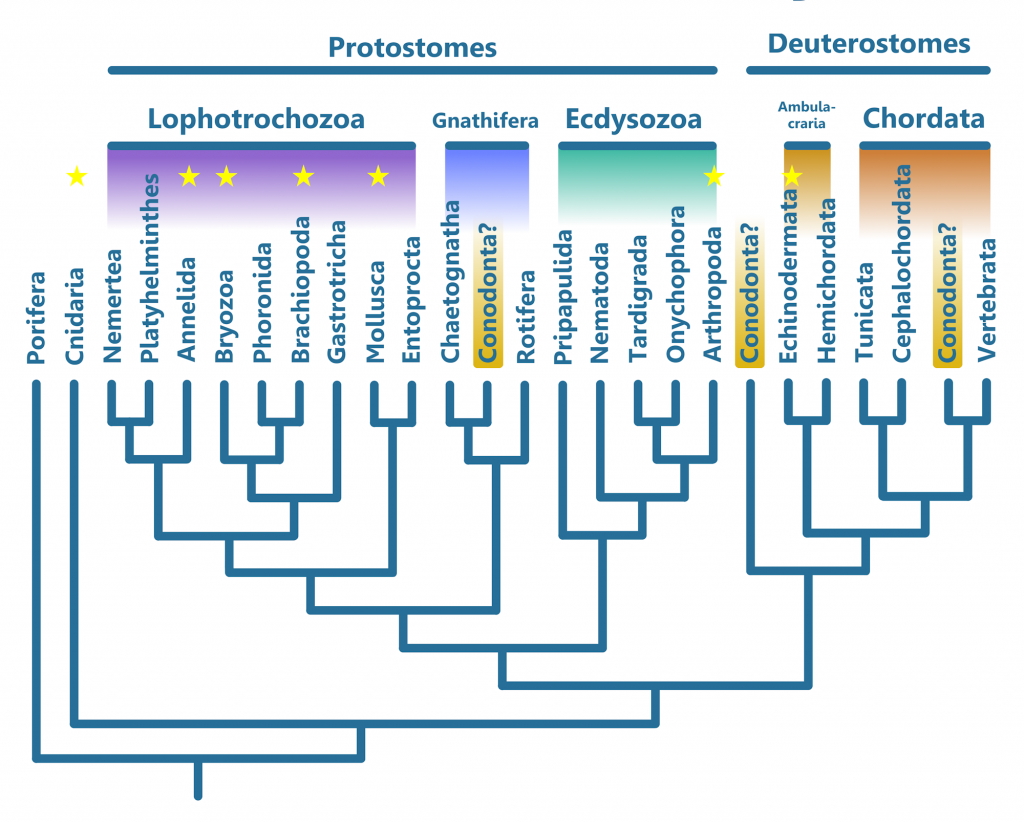
The other main theory as to how conodonts should be classified, as stated above, places them as sister taxa to the Chaetognaths. The Protoconodonts have been suspected to be Chaetognaths for a long time (6), share no tissues with the Paraconodonts and Euconodonts, and when found in clusters, appear in the same orientations as one would expect for Chaetognaths (9). It is thought that they may even have given rise to Chaetognaths in the first place; the big question is whether they were related to the true Conodonta or not.
Other than Craniates and Conodonts, Chaetognaths are the only animals with fin ray-like structures. Though Chaetognaths were once seen as Deuterostomes, like Chordates and Ambulacrarians (the group that includes Echinodermata and Hemichordata, which are not Chordates), they are now classified as Protostomes (20), like all other animals excluding Porifera (sponges) and Cnidaria (Coral, jellyfish, comb jellies, and their close relatives). Their fins are located pectorally and posteriorally, and are symmetrical. While the longitudinal organ in Conodonts could be seen as a gut, all conodont fossils that preserve soft tissues (the three being Clydagnathus, Promissum, and the possibly venomous (7), heavily built, macrophagous predator (8) Panderodus) lack the characteristic partitions that differentiate the head, body, and tail of Chaetognaths (4). If the cone-shaped structures found in conodonts are eyes, than they would be similarly placed to the eyes of Chaetognaths as well as the Craniates. In this view, the Protoconodonts gave rise to both the Chaetognaths and the Conodonta, despite having none of the tissues of which conodont elements were made. This is expressed in the phylogeny below.
Discussion of Conodont Features and Classification
While Chaetognath affinities seem like a pretty good explanation for the Conodonts, there are some drawbacks to this solution. For instance, as mentioned above, Chaetognaths have symmetrical fins on the sides of their bodies, instead of the dorsoventral fins of Chordates. The Clydagnathus specimens, the only fossils to preserve both the upper and lower halves of the entire Conodont, do not appear to preserve symmetrical fins; whether the lower half of the fossil was compressed dorsally or laterally is unclear (6). It is possible that Clydagnathus had symmetrical, lateral fins, but that they were distorted during fossilization and now appear asymmetrical, but the longitudinal organ passes straight through this region without evidence of distortion (6) (See Figure 5). This would suggest that the fins of Conodonts were asymmetrical, in line with Chordate fins. This would also infer a dorsoventral orientation of the fins.
Another piece of evidence against Chaetognath affinities of Conodonts is that they share no tissues within their grasping elements (2); this suggests that Protoconodonts were not part of Conodonta, too.
Finally, the longitudinal organ possessed by Conodonts must be interpreted differently in different modes of Conodont classification, because Chaetognaths do not possess notochords (4, 6). While traditionally seen as a notochord by those classifying Conodonts as Chordates, those who place the Conodonta next to Chaetognatha must view it as a gut (6). Additionally, in the Chordate theory this structure could represent a gut, but this is not as commonly agreed upon (4). This organ originates almost directly behind the head (4), made up of two parallel lines that run down the entire body, through the anterior tips of the myomeres. By the time they get to the tail, the lines appear as one, and end slightly below the tip of the tail (6). Interestingly, this is powerful evidence against it being a gut in both Chaetognaths and Chordates, because in both groups the anus lies anterior to the tip of the tail. Cephalochordates, on the other hand, have a notochord that follows the same path as the longitudinal organ in Conodonts, suggesting that that is the identity of this structure (See figure 5). This is again evidence for a Chordate placement of Conodonta.
Other Important Information
Studies involving the lamellae of Conodont crowns suggests that at least some Late Devonian Conodonts related to Clydagnathus lived for roughly a month (17). Additionally, Promissum pulchrum, mentioned above because of its soft tissue preservation, is thought ho have been an efficient cruiser, but not capable of short bursts of speed, because of the nature of its muscle fibers. (16).
Conodonts at Seven Stars
As mentioned above, there were almost certainly multiple genera of Conodonts at Seven Stars. They likely were important parts of the ecosystem, feeding on other plankton.
Conodonta indet.
Conodont elements can be identified in the figure at right. The conodont that grew them likely grew a couple centimeters long. This element may represent multiple smaller elements fused together, as is common in rocks that were pressurized heated in the past by geological processes.
Works cited
(1) Dzik, J. 2002. Emergence and collapse of the Frasnian conodont and ammonoid communities in the Holy Cross Mountains, Poland. Acta Palaeontologica Polonica 47 (4): 565–650. https://app.pan.pl/archive/published/app47/app47-565.pdf
(2) Sweet, Walter & Donoghue, Philip. (2001). Conodonts: Past, present, future. Journal of Paleontology. 75. 1174-1184. 10.1017/S0022336000017224. https://www.researchgate.net/publication/305883634_Conodonts_Past_present_future
(3) von Bitter, P.H. & Norby, R.D. 1994 10 15: Fossil epithelial cell imprints as indicators of conodont biology. Lethaia 27, pp. 193-198. Oslo. ISSN 0024-1 164 https://www.idunn.no/doi/pdf/10.1111/j.1502-3931.1994.tb01408.x
(4) Turner S., Burrow C. J., Schultze H.-P., Blieck A., Reif W.-E.†, Rexroad C. B., Bultynck P. & Nowlan G. S. 2010. — False teeth: conodont-vertebrate phylogenetic relationships revisited. Geodiversitas 32 (4): 545-594 www.vliz.be/imisdocs/publications/294835.pdf
(5) Iannicelli, Michael. (2018). Explaining the Crude and Simple Mechanics of Parasitic Feeding by Conodonts. Journal of Oceanography and Marine Research. 06. 10.4172/2572-3103.1000177. https://www.researchgate.net/publication/326231188_Explaining_the_Crude_and_Simple_Mechanics_of_Parasitic_Feeding_by_Conodonts
(6) Briggs, Derek E. G., Clarkson, Euan N. K. & Aldridge, Richard J. 1983 01 15: The conodont animal. Lethaia, Vol. 16, pp. 1-14. Oslo. ISSN 0024-1164 https://www.idunn.no/doi/epdf/10.1111/j.1502-3931.1983.tb01993.x
(7) Szaniawski, H. 2009. The earliest known venomous animals recognized among conodonts. Acta Palaeontologica Polonica 54 (4): 669–676. doi:10.4202/app.2009.0045 https://www.app.pan.pl/archive/published/app54/app20090045.pdf
(8) Murdock, D.J.E. and Smith, M.P. (2021), Panderodus from the Waukesha Lagerstätte of Wisconsin, USA: a primitive macrophagous vertebrate predator. Pap Palaeontol, 7: 1977-1993. https://doi.org/10.1002/spp2.1389. https://onlinelibrary.wiley.com/doi/full/10.1002/spp2.1389
(9) Vannier, Jean & Steiner, Michael & Renvoisé, Elodie & Hu, Shixue & Casanova, J.-P. (2006). Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proceedings. Biological sciences / The Royal Society. 274. 627-33. 10.1098/rspb.2006.3761. https://www.researchgate.net/publication/6549148_Early_Cambrian_origin_of_modern_food_webs_evidence_from_predator_arrow_worms
(10) N. Goudemand, M.J. Orchard, S. Urdy, H. Bucher, P. Tafforeau, Synchrotron-aided reconstruction of the conodont feeding apparatus and implications for the mouth of the first vertebrates, Proc. Natl. Acad. Sci. U.S.A. 108 (21) 8720-8724,https://doi.org/10.1073/pnas.1101754108 (2011).
https://www.pnas.org/doi/10.1073/pnas.1101754108
(11) Jones, David & Evans, Alistair & Siu, Karen & Rayfield, Emily & Donoghue, Philip. (2012). The sharpest tools in the box? Quantitative analysis of conodont element functional morphology. Proceedings. Biological sciences / The Royal Society. 279. 2849-54. 10.1098/rspb.2012.0147. https://www.researchgate.net/publication/221895928_The_sharpest_tools_in_the_box_Quantitative_analysis_of_conodont_element_functional_morphology
(12) Purnell, Mark & Jones, David. (2012). Quantitative analysis of conodont tooth wear and damage as a test of ecological and functional hypotheses. Paleobiology. 38. 605-626. 10.2307/41684303. https://www.researchgate.net/publication/262085316_Quantitative_analysis_of_conodont_tooth_wear_and_damage_as_a_test_of_ecological_and_functional_hypotheses
(13) Epstein, Anita G. et al. (1975). Conodont color alteration; an index to organic metamorphism. Geological Survey. https://permanent.fdlp.gov/gpo89251/report.pdf
(14) Dzik, J. (1991). Evolution of oral apparatuses in the conodont chordates. Acta
Palaeontologica Polonica 36, 3, 265-32 https://www.app.pan.pl/archive/published/app36/app36-265.pdf
(15) Wilson, K. A. (2014). Field guide to the Devonian fossils of New York (3rd ed.). Paleontological Research Institution.
(16) Gabbott, S.E.; R. J. Aldridge; J. N. Theron. (1995). A giant conodont with preserved muscle tissue from the Upper Ordovician of South Africa. Nature. 374 (6525): 800–803. doi:10.1038/374800a0. https://www.academia.edu/13746759/A_giant_conodont_with_preserved_muscle_tissue_from_the_Upper_Ordovician_of_South_Africa
(17) Świś, Przemysław. (2018). Population dynamics of the Late Devonian conodont Alternognathus calibrated in days. Historical Biology. 1-9. 10.1080/08912963.2018.1427088. https://www.researchgate.net/publication/322550688_Population_dynamics_of_the_Late_Devonian_conodont_Alternognathus_calibrated_in_days
(18) Terrill DF, Jarochowska E, Henderson CM, Shirley B, Bremer O. Sr/Ca and Ba/Ca ratios support trophic partitioning within a Silurian conodont community from Gotland, Sweden. Paleobiology. 2022;48(4):601-621. doi:10.1017/pab.2022.9 https://www.cambridge.org/core/journals/paleobiology/article/srca-and-baca-ratios-support-trophic-partitioning-within-a-silurian-conodont-community-from-gotland-sweden/AD2822CD8671922CAB8E821D193F2BA8
(19) Conway Morris, S. (1990). Typhloesus wellsi (Melton and Scott, 1973), a bizarre metazoan from the Carboniferous of Montana, U. S. A. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 327(1242), 595–624. https://doi.org/10.1098/rstb.1990.0102 (Original work published April 12, 1990 https://royalsocietypublishing.org/doi/10.1098/rsbl.2022.0179
(20) Marlétaz, Ferdinand & Peijnenburg, K. & Goto, Taichiro & Satoh, Nori & Rokhsar, Daniel. (2019). A New Spiralian Phylogeny Places the Enigmatic Arrow Worms among Gnathiferans. Current Biology. 29. 10.1016/j.cub.2018.11.042. https://www.cell.com/current-biology/pdfExtended/S0960-9822(18)31541-0
These resources were used in the reconstructions in this post, but were not referenced in the text.
Gabbott, Sarah & Browning, Claire & Theron, J.N. & Whittle, Rowan. (2016). The late Ordovician Soom Shale Lagerstätte: An extraordinary post-glacial fossil and sedimentary record. Journal of the Geological Society. 174. jgs2016-076. 10.1144/jgs2016-076. https://www.lyellcollection.org/doi/10.1144/jgs2016-076
Lowe, Christopher & Clarke, Nat & Medeiros, Daniel & Rokhsar, Daniel & Gerhart, John. (2015). The deuterostome context of chordate origins. Nature. 520. 456-65. 10.1038/nature14434. https://www.researchgate.net/publication/275360662_The_deuterostome_context_of_chordate_origins
Topper, Timothy & Betts, Marissa & Dorjnamjaa, Dorj & Li, Guoxiang & Li, Luoyang & Gundsambuu, Altanshagai & Enkhbaatar, Batkhuyag & Skovsted, Christian. (2022). Locating the BACE of the Cambrian: Bayan Gol in southwestern Mongolia and global correlation of the Ediacaran–Cambrian boundary. Earth-Science Reviews. 229. 104017. 10.1016/j.earscirev.2022.104017. https://www.researchgate.net/publication/359627144_Locating_the_BACE_of_the_Cambrian_Bayan_Gol_in_southwestern_Mongolia_and_global_correlation_of_the_Ediacaran-Cambrian_boundary
Tomita, T. (2015). Pectoral Fin of the Paleozoic Shark, Cladoselache: New Reconstruction Based on a Near-Complete Specimen. Journal of Vertebrate Paleontology. https://www.semanticscholar.org/paper/Pectoral-Fin-of-the-Paleozoic-Shark%2C-Cladoselache%3A-Tomita/58f347cd34a47eaf8da3e05aafe71d71dc316baf
Xianren, Shan & Zhu, Min & Zhao, Wenjin & Pan, Zhaohui & Wang, Pingli & Gai, Zhikun. (2020). A new genus of sinogaleaspids (Galeaspida, stem-Gnathostomata) from the Silurian Period in Jiangxi, China. PeerJ. 8. e9008. 10.7717/peerj.9008. https://pdfs.semanticscholar.org/e542/e8a81ccbbf8b21636be84595dee2d3e649b1.pdf